Publications
»2026«
Influence of lanthanide oxide supports on the performance of barium-promoted cobalt catalysts for ammonia synthesis, „Catalysis Today”
Patkowski W, Zybert M, Ulkowska U, Ronduda H, Bulejak W, Lemańska M, Albrecht A, Moszyński D, Fidler A, Dłużewski P, Raróg-Pilecka W., Influence of lanthanide oxide supports on the performance of barium-promoted cobalt catalysts for ammonia synthesis, „Catalysis Today”, 2026, t.463, s. 1–12.
DOI:
https://doi.org/10.1016/j.cattod.2025.115611.
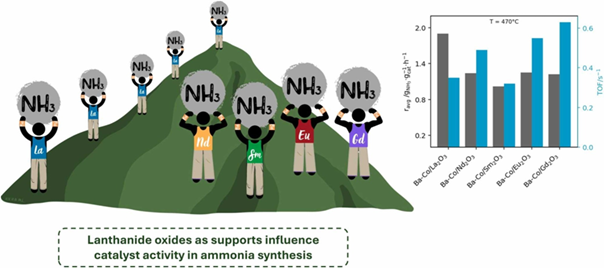
Abstract
Developing efficient ammonia synthesis catalysts is key to reducing energy consumption and improving sustainability. This study explores barium-promoted cobalt catalysts supported on lanthanide oxides (La2O3, Nd2O3, Sm2O3, Eu2O3, Gd2O3) to understand how support choice influences catalytic performance. The catalysts were characterised using techniques such as X-ray powder diffraction (XRPD), high-resolution transmission electron microscopy (HRTEM), and temperature-programmed desorption (H2-TPD, CO2-TPD). Testing under industrially relevant conditions (400–470°C, 6.3 MPa, H2/N2 = 3) revealed that lanthanide oxides strongly affect catalysts’ activity, reducibility, and hydrogen adsorption. Among the tested catalysts, the La2O3-supported system exhibited the highest ammonia synthesis activity, likely due to its favorable hydrogen sorption properties and larger active phase surface area available for hydrogen (31 m²·gCo⁻¹). These findings highlight the potential of lanthanide oxides as supports and the importance of barium as a promoter in cobalt-based catalysts for ammonia synthesis.
Highlights
- Lanthanide oxides as supports influence catalyst activity in ammonia synthesis.
- Ba-Co/La₂O₃ shows highest activity due to H₂ activation and larger active area.
- Support type impacts Co reducibility, H₂ adsorption, and active site formation.
Keywords
Ammonia synthesis; Barium promotion; Cobalt catalysts; Lanthanide oxides
Role of the active metal (Fe, Co and Ni) on the activity of Nd2O3-supported catalysts for ammonia synthesis, „Catalysis Today”
Ronduda H., Lemańska M., Patkowski W., Ostrowski A., Sobczak K., i Raróg-Pilecka W., Role of the active metal (Fe, Co and Ni) on the activity of Nd2O3-supported catalysts for ammonia synthesis, „Catalysis Today”, 2026, t.461, s. 1–11.
DOI:
https://doi.org/10.1016/j.cattod.2025.115535
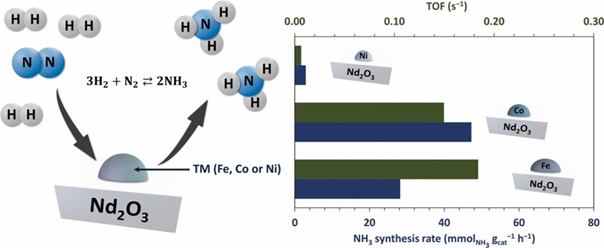
Abstract
Nd2O3-supported Fe, Co, and Ni monometallic catalysts were synthesised and used in the ammonia synthesis reaction. The effect of active metal on the physicochemical and catalytic properties was investigated using, e.g., TPR, TPD, XRD, and STEM. The results showed that the kind of active metal used is the factor determining the catalytic activity in ammonia synthesis. The highest ammonia formation rate was exhibited by Co/Nd2O3, whereas the highest intrinsic reaction rate (reflected as the TOF value) was shown by Fe/Nd2O3. Ni/Nd2O3 showed almost no activity in ammonia synthesis. The high ammonia formation rate observed for Co/Nd2O3 was attributable to the well-distributed Co nanoparticles (NPs) on the Nd2O3 support. The Co NPs were less prone to sintering than Fe NPs under reaction conditions. Thus, although showing lower TOF than Fe NPs, the better distribution of Co NPs resulted in an improved ammonia formation rate. These findings highlight the importance of rational catalyst design for improving ammonia synthesis efficiency.
Highlights
- Nd2O3-supported Fe, Co, and Ni monometallic catalysts for NH3 synthesis.
- Superior NH3 synthesis performance of the Fe/Nd2O3 and Co/Nd2O3 catalysts.
- Excellent resistance to sintering of Co and Ni nanoparticles.
Keywords
Ammonia synthesis; Supported catalysts; Iron; Cobalt; Nickel
»2025«
Enhanced ammonia synthesis over barium cerate-supported cobalt catalyst by rare-earth element doping, „Catalysis Today”
Ronduda H., Lemańska M., Ulkowska U., Patkowski W., Ostrowski A., Sobczak K., i Raróg-Pilecka W., Enhanced ammonia synthesis over barium cerate-supported cobalt catalyst by rare-earth element doping, „Catalysis Today”, 2025, t.456, s. 1–10.
DOI:
https://doi.org/10.1016/j.cattod.2025.115342
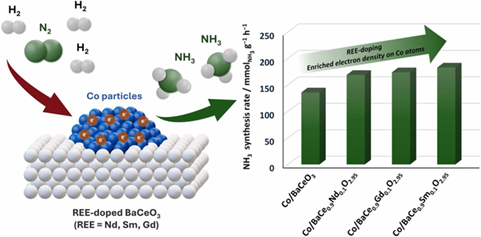
Abstract
A series of BaCeO3 doped with various rare-earth elements (REE = Nd, Sm, Gd) were synthesised and used as the supports for cobalt catalysts for ammonia synthesis. The effects of rare-earth dopant type and concentration on the physicochemical properties and catalytic activities were studied using, e.g., XRD, STEM-EDX, and TPD techniques. Catalyst testing revealed that the optimal doping concentration was 10 mol%, regardless of the rare-earth ion. Samarium was identified as the most effective dopant, followed by gadolinium and neodymium. The superior performance of the Co/BaCe0.9REE0.1O3–δ catalysts was due to the incorporation of REE dopant into the BaCeO3 structure, which increased the electron density, enabling efficient electron transfer from the support to the Co surface. This, in turn, facilitated the N2 dissociative adsorption, recognised as the rate-determining step (RDS) of ammonia synthesis. In addition, the catalysts were characterised by favourable hydrogen adsorption properties (co-existence of weak and strong adsorption sites), contributing to the effective hydrogen activation under the reaction conditions. This study provides an effective approach for designing cobalt catalysts supported on perovskites, demonstrating their great potential as next-generation catalysts for ammonia synthesis.
Highlights
- Co catalysts supported on REE-doped BaCeO3 (REE = Nd, Sm, Gd) for NH3 synthesis.
- Tuneable acid-base properties of BaCeO3 supports by REE doping strategy.
- Strong influence of support basicity on catalyst performance.
- Superior performance of Co supported on BaCe0.9Sm0.1O3–δ catalyst.
Keywords
Ammonia synthesis; Cobalt catalysts; Perovskite supports; Doping; Rare-earth elements
Understanding the role of caesium additive in cobalt catalysts for ammonia synthesis, „Journal of Thermal Analysis and Calorimetry”
Ronduda H., Zybert M., Patkowski W., Lemańska M., Ostrowski A., Sobczak K., i Raróg-Pilecka W., Understanding the role of caesium additive in cobalt catalysts for ammonia synthesis, „Journal of Thermal Analysis and Calorimetry”, 2025.
DOI:
https://doi.org/10.1007/s10973-025-14354-x
Abstract
Ammonia (NH3) is the second most produced chemical globally and is one of the largest chemical industries by volume. However, although being well-established, it requires high temperature and pressure conditions, which result in a large energy consumption and carbon dioxide emissions. To address this issue, the development of novel catalysts with high activity under mild reaction conditions has become a prominent area of research. Although the effect of Cs as the promoter for Fe and Ru catalysts has been well described, the effect of Cs doping on the Co catalyst performance in ammonia synthesis has not been well studied yet. Here, we studied the effect of Cs doping on the physicochemical properties and activity of the Co catalysts. It was found that caesium doping was detrimental to the catalytic activity. The ammonia synthesis rate decreased with the increasing amount of caesium added. The Cs species were not thermally stable and evaporated from the surface of the catalysts during the reductive activation. In addition, the Cs doping contributed to the catalyst sintering, resulting in the irreversible loss of active sites. This was the primary reason for the observed poor activity of the Cs-doped Co catalysts in ammonia synthesis.
Keywords
Ammonia; Ammonia synthesis; Catalyst; Cobalt catalyst; Caesium
»2024«
Toward green ammonia synthesis - exploring the influence of lanthanide oxides as supports on the cobalt catalysts properties, „Journal of CO2 Utilization”
Patkowski W., Zybert M., Ronduda H., Albrecht A., Moszyński D., Fidler A., Dłużewski P., Mierzwa B., i Raróg-Pilecka W., Toward green ammonia synthesis – exploring the influence of lanthanide oxides as supports on the cobalt catalysts properties, „Journal of CO2 Utilization”, 2024, t.80, s. 1–13.
DOI:
https://doi.org/10.1016/j.jcou.2024.102699\
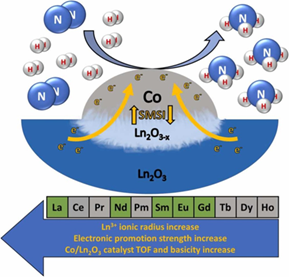
Abstract
Nowadays, ammonia is viewed as the prospective green hydrogen fuel carrier. Yet, there still is a demand for active, energy-efficient NH3 synthesis catalysts to make ammonia use economically viable. This paper studies and discusses lanthanide oxide-supported cobalt catalysts for low-pressure ammonia synthesis, which are intended to be a model for prototyping systems alternative to ruthenium-based catalysts. In the Co/Ln2O3 system, lanthanide oxide is a support and an electronic promoter. The electron density on the active phase’s surface is increased through Strong Metal-Support Interactions and the formation of oxygen-deficient, Ln2O3−x species capable of electron donation. In the systems, electronic promotion strength depends on the Ln3+ cation and the support conductivity, catalyst Turnover Frequency and basicity increase exponentially with the increase of the Ln3+ ionic radius. These dependencies enable the design of novel cobalt catalysts of specific properties and outline the prospects for their further development.
Highlights
- Ammonia synthesis cobalt catalysts deposited on lanthanide oxides were synthesised.
- Lanthanide oxide supports act as electronic promoters of the metallic active phase.
- In Co/Ln2O3 systems Ln2O3−x strong electron-donating sites are formed due to SMSI.
- Promotion influence of Ln2O3 depends on the ionic radius of the Ln3+ cation.
- Growth of Ln3+ radius causes an exponential increase of catalyst TOF and basicity.
Keywords
Ammonia synthesis; Cobalt; Lanthanide oxide; SMSI; Electronic promotion
Cobalt catalysts for COx-free hydrogen production: Effect of catalyst type on ammonia decomposition in gliding discharge plasma reactor, „Journal of CO2 Utilization”
Ronduda H., Młotek M., Góral W., Zybert M., Ostrowski A., Sobczak K., Krawczyk K., i Raróg-Pilecka W., Cobalt catalysts for COx-free hydrogen production: Effect of catalyst type on ammonia decomposition in gliding discharge plasma reactor, „Journal of CO2 Utilization”, 2024, t.82, s. 1–12.
DOI:
https://doi.org/10.1016/j.jcou.2024.102755
Abstract
Hydrogen is considered the cleanest, most environmentally friendly fuel and energy carrier required for the gradual decarbonisation of many industrial sectors. Using ammonia as a Cox-free source of hydrogen is the most reasonable and most applicable method. This paper studies the properties and activity of cobalt catalysts in the ammonia decomposition reaction using a plasma-catalytic system. The effect of catalyst type (supported versus bulk) was evaluated. The catalysts were examined using XRD, STEM-EDX, and sorption techniques (N2 physisorption, TGA-TPR, H2-TPD, CO2-TPD) to reveal the influence of physicochemical properties of these two types of catalysts on the efficiency of NH3 decomposition in the plasma-catalytic process using a gliding discharge plasma. The results disclose that the supported-type catalyst (Ba-Co/CeO2) decomposed NH3 more effectively than the bulk-type catalyst (Co/Ce/Ba). At discharge power of 300 W and flow rate of 180 dm3 h–1 of NH3:N2 mixture (50/50 vol%), the ammonia conversion over the Ba-Co/CeO2 catalyst was 70%, whereas over the Co/Ce/Ba catalyst it was only 21%. The favourable performance of the supported-type catalyst was attributed to a more thermally stable surface area compared with the bulk-type catalyst. Smaller and more stable cobalt nanoparticles (NPs) with numerous weak hydrogen adsorption sites were also seen. Meanwhile, the strong basic sites were generated, improving the electron-donating ability of the surface active sites. High ammonia conversion and relatively low-energy consumption of the plasma-catalytic ammonia decomposition over Ba-Co/CeO2 make it suitable for practical hydrogen production applications, such as fuel cells and hydrogen storage.
Highlights
- Supported and bulk cobalt catalysts were synthesised and tested in NH3 decomposition using gliding discharge plasma.
- Supported catalyst decomposed ammonia more effectively than bulk catalyst.
- High performance of supported catalyst was ascribed to thermal stability, favourable H2 adsorption, and strong basicity.
- NH3 conversion over supported catalyst achieved 96% with energy consumption of 3.9 kWh m–3 H2 at 500 W discharge power.
Keywords
Ammonia; Ammonia decomposition; Plasma-catalyst interactions; Catalyst; Cobalt catalyst
Elucidating the role of potassium addition on the surface chemistry and catalytic properties of cobalt catalysts for ammonia synthesis, „RSC Advances”
Ronduda H., Zybert M., Patkowski W., Ostrowski A., Sobczak K., Moszyński D., i Raróg-Pilecka W., Elucidating the role of potassium addition on the surface chemistry and catalytic properties of cobalt catalysts for ammonia synthesis, „RSC Advances”, 2024, t.14, s. 23095–23108.
DOI:
https://doi.org/10.1039/d4ra04517c
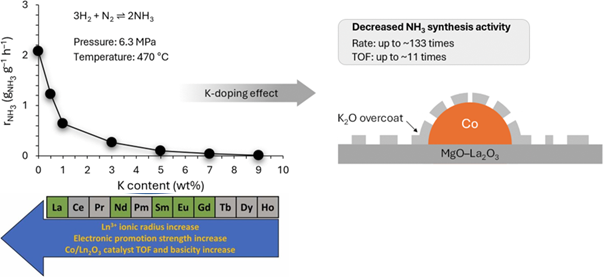
Abstract
The ammonia synthesis process produces millions of tons of ammonia annually needed for the production of fertilisers, making it the second most produced chemical worldwide. Although this process has been optimised extensively, it still consumes large amounts of energy (around 2% of global energy consumption), making it essential to improve its efficiency. To accelerate this improvement, research on catalysts is necessary. Here, we studied the role of potassium in ammonia synthesis on cobalt catalysts and found that it was detrimental to the catalytic activity. It was shown that, regardless of the amount of introduced K, the activity of the K-modified catalysts was much lower than that of the undoped catalyst. K was found to be in the form of oxide; however, it was unstable and reducible to metallic K, which easily volatilised from the catalyst surface under activation conditions. In addition, potassium doping resulted in the sintering of the catalyst, the decrease in the surface basicity, and contributed to the loss of the active sites, mainly due to the coverage of Co surface by residual K species. K-doped cobalt catalysts for ammonia synthesis: the location, state and effect of potassium dopant on the surface chemistry and catalytic properties.
Improving the catalytic performance of Co/BaCeO3 catalyst for ammonia synthesis by Y-modification of the perovskite-type support, „RSC Advances”
Zybert M., Ronduda H., Patkowski W., Ostrowski A., Sobczak K., i Raróg-Pilecka W., Improving the catalytic performance of Co/BaCeO3 catalyst for ammonia synthesis by Y-modification of the perovskite-type support, „RSC Advances”, 2024, t.14, s. 36281–36294.
DOI:
https://doi.org/10.1039/d4ra06251e
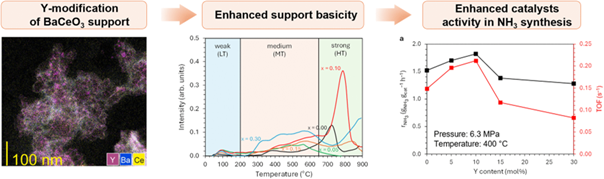
Abstract
Y-modified perovskite-type oxides BaCe1−xYxO3−δ (x = 0–0.30) were synthesised and used as supports for cobalt catalysts. The influence of yttrium content on the properties of the support and catalyst performance in the ammonia synthesis reaction was examined using PXRD, STEM-EDX, and sorption techniques (N2 physisorption, H2-TPD, CO2-TPD). The studies revealed that the incorporation of a small amount of yttrium into barium cerate (up to 10 mol%) increased specific surface area and basicity. The catalyst testing under conditions close to the industrial ones (T = 400–470°C, p = 6.3 MPa, H2/N2 = 3) showed that the most active catalyst was deposited on a support containing 10 mol% Y. The NH3 synthesis reaction rate was 15–20% higher than that of the undoped Co/BaCeO3 catalyst. The activity of the catalysts decreased with further increasing Y content in the support (up to 30 mol%). However, all the studied Co/BaCe1−xYxO3−δ catalysts exhibited excellent thermal stability, over 240 h of operation. The particularly beneficial properties of the catalyst containing 10 mol% of Y were associated with the highest basicity of the support surface, favourable adsorption properties (suitable proportion of weakly and strongly hydrogen-binding sites), and preferred size of cobalt particles (60 nm). The Co/BaCe0.90Y0.10O3−δ catalyst showed better ammonia synthesis performance compared to the commercial iron catalyst (ZA-5), giving prospects for process reorganisation towards energy-efficient ammonia production.
The beneficial effect of Y3+ ions incorporated into BaCeO3 support structure stems from the strengthening of the electron-donating ability, i.e., better charge transfer from the support to the active metal, enhancing N2 dissociation.
Faculty of Chemistry, Warsaw University of Technology
Department of Chemical Technology
e-mail: wioletta.pilecka@pw.edu.pl
LinkedIn: linkedin.com/in/wioletta-raróg-pilecka-1bb602311
Koszykowa 75 St.
00-662 Warsaw, Poland
